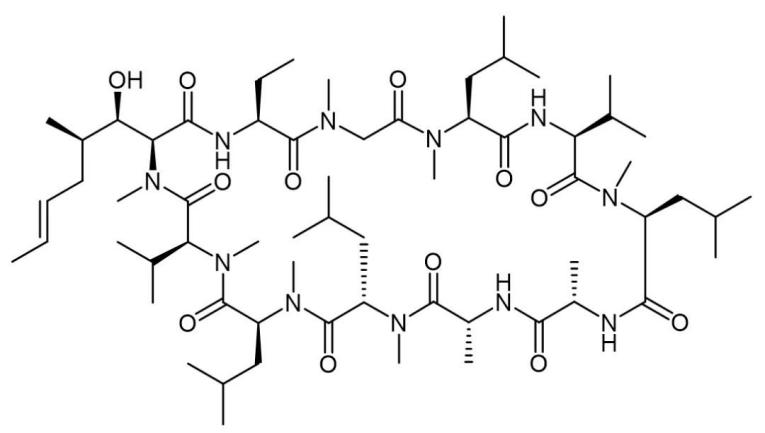
As early as the 80s of the last century (1983), the exploration of oral delivery of peptides has achieved a historic breakthrough through cyclosporine A (Fig.1). This natural peptide can achieve high oral bioavailability and is therefore developed as an oral formulation.
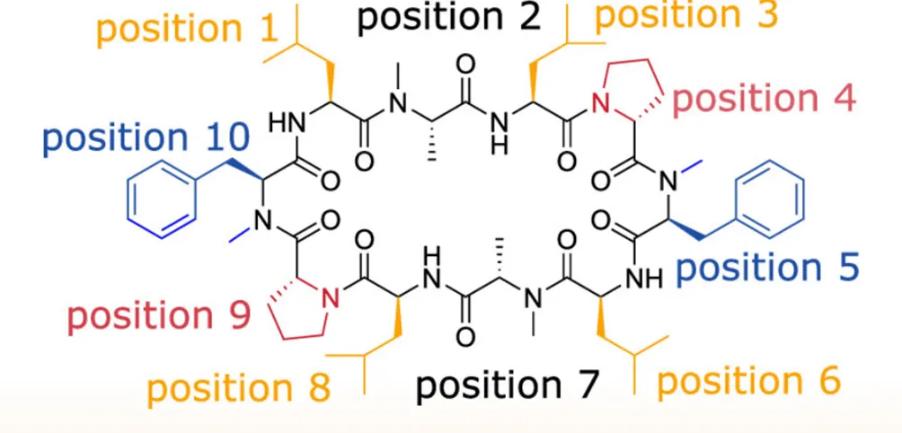
However, the oral delivery of Cyclosporine A has stimulated people’s enthusiasm for the development of oral peptides, but the key to completely opening the door to oral peptides has not been fully presented, and people are still searching for the realization of oral bioavailability of peptides. In addition to the stability of peptides and their resistance to degradation by a variety of enzymes, how to cross the cell membrane is also one of the focuses of research. Researchers from the University of ETH in Switzerland recently simulated the molecular dynamics of peptide crossing the cell membrane using a flexible decacyclic peptide (Fig.2).
In contrast to straight-chain peptides, which are too flexible, cyclic peptides have what are known as chameleon properties. Chameleons change skin color based on the environment, while cyclic peptides can change the molecular conformation based on the polarity of the environment. This property depends on the conversion of intramolecular and intermolecular hydrogen bonds in the cyclic peptide molecule.
In the so-called Open conformation of investigational peptide drugs, the hydrogen-bonded atoms of cyclic peptides are exposed to a polar environment (e.g., in blood or cytoplasm) to form hydrogen bonds with their counterparts in solvents, or with polar solvent molecules. But when the environment becomes non-polar (e.g., inside a cell membrane), cyclic peptides can instead adopt the so-called Closed conformation, wrapping themselves up and encapsulating themselves by forming intramolecular hydrogen bonds, encapsulating polar groups inside the molecule and exposing the non-polar structure to surface of the molecule, thereby reducing the polar area and the desolvation energy (the amount of energy required for the solute to be removed from the water or other solvents). This chameleon property of cyclic peptide is helpful in the development of oral peptide formulations because it can balance good water and fat solubility, which favors increasing cell permeability.
The Closed state is generally considered to be the predominant permeability state of cyclic peptides. However, it is a dynamic process that is controlled by a variety of factors such as sequence, molecular size, hydrophobic surface area, etc. Through molecular dynamics simulation, the researchers simulated the process of cyclic peptide molecules crossing the cell membrane through passive osmosis into the cell, highlighting the importance of flexible conformation for cyclic peptide membrane permeation, and dividing it into four steps (Fig.3):
- Specific peptide side-chain residues can act as molecular anchors to establish the initial contact of cyclic peptides with the cell membrane before the peptide molecule is inserted into the cell membrane.
- After anchoring to the cell membrane, the peptide molecule jumps directly into it, inserting itself into the interface between the polar head and non-polar tail regions of the cell membrane. In this process, the cyclic peptide exhibits two potential directions of arrangement, and the open state takes orientation A and B, and preference orientation A is preferred. Closed cyclic peptides take orientation B only.
- The Open state transitions to the Closed state, increasing the chance of it penetrating. At this time, the chameleon nature of the peptide molecules is very important for them to move from a polar environment to a non-polar environment. It is difficult for the cyclic peptide in the open state to achieve the subsequent diffusion process.
- Only cyclic peptide molecules in the closed state are likely to diffuse from this polar/non-polar mixed environment on the cell membrane surface through the non-polar lipid membrane leaflet and spread to the polar/non-polar interface on the other side. Through the anchoring and flipping mechanism, the final transmembrane journey is achieved.
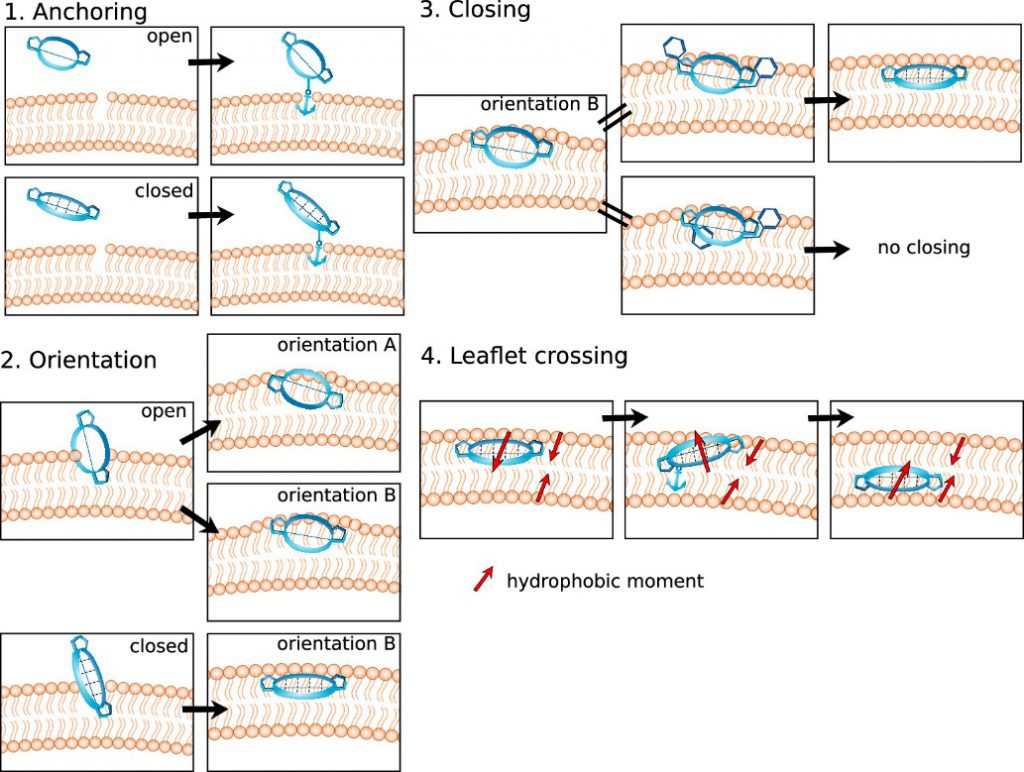
Step 1
Simulated molecular dynamics showed that the anchor residues of the Open conformation and the Closed conformational cyclic peptide were different, due to the fact that the degree of contact of the residues was not the same in the two conformations. Given that the anchoring of amino acids is peptide conformation-dependent, characterizing the conformational behavior of cyclic peptides is critical for rational peptide drug design. Understanding the main conformations of peptides before they enter the cell membrane and the corresponding residue exposure will help optimize the design of membrane-permeable cyclic peptides. This mode of membrane permeability initiated by side chain anchoring of amino acid residues can be generalized to other molecular modalities other than peptides, such as increasing the membrane permeability of molecules by means of moderate aliphatic chain conjugation.
Step 2
The peptide is inserted into the membrane and parallels itself to the membrane plane. If the insertion occurs in a molecule with an open conformation, the peptide may take two directions of arrangement, A and B. However, if the peptide insertion is already in a closed conformation (pre-folded), only direction B is observed. This can also be useful for the design of other macrocyclic peptides, especially those with continuous hydrophobic surfaces. Only orientation B favors the conversion of peptides from the Open to Closed conformation.
Step 3
The change of conformation from the open state to the closed state is a critical process that has an important impact on the subsequent intramembrane diffusion. This conformational transition not only changes the spatial structure of the molecule, but also significantly affects its interaction with the membrane environment, thus determining the movement mode and diffusion efficiency of the molecule in the membrane.
Step 4
The peptide molecule that enters the cell membrane must expand to the other side of the membrane (lower leaflet). As the final step of crossing the membrane, the peptide molecule needs to go through a similar process as the first step, that is, its amino acid residues need to be anchored to the polar head of the lower leaflet, and this process also involves the reversal of the direction of the cyclic peptide, resulting in a change in the direction of the hydrophobic moment.
Simulations have shown that the effects of amino acids (e.g., leucine, phenylalanine, proline) may be highly correlated with the peptide sequence, depending on the position of the amino acid in the peptide as well as other residues. The goal of this molecular dynamics simulation of the cyclic peptide permeation pathway is to improve the bioavailability of peptides, especially oral bioavailability, and to develop more peptide drugs that can be delivered orally.
References
Linker, Stephanie M., et al., Lessons for Oral bioavailability: how Conformationally flexible cyclic peptides enter and cross lipid membranes. Journal of medicinal chemistry 66.4 (2023): 2773-2788.