What is Trofinetide?
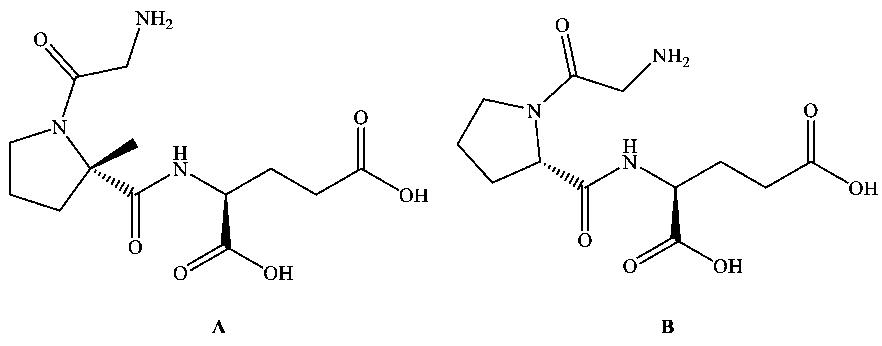
Trofinetide is a synthetic glycine-proline-glutamic acid (GPE) tripeptide analogue derived from the N-terminal sequence of insulin-like growth factor 1 (IGF-1). It is primarily used to treat Rett syndrome (RTT), a rare inherited neurodevelopmental disorder that primarily affects women and is usually caused by mutations in the MECP2 gene.
Trofinetide works by reducing neuroinflammation and supporting synaptic function. Its mechanism of action includes enhancing synaptic activity, restoring synaptic structure, inhibiting the effects of inflammatory compounds in the brain, increasing antioxidant responses, reducing injury-induced apoptosis, and increasing the presence of IGF-1 in the central nervous system. In addition, Trofinetide has also been found to be able to inhibit the production of inflammatory cytokines and inhibit the overactivation of microglia and astrocytes.
Trofinetide is the first FDA-approved drug for the treatment of Rett syndrome in adults and children 2 years of age and older. In clinical trials, Trofinetide has shown significant improvements in the core symptoms of Rett syndrome, such as communication, behavior, and motor function. However, although its efficacy has been validated, its exact mechanism of action needs further research. Trofinetide is an innovative therapy for Rett syndrome that improves the neurological function and structure of patients through multiple mechanisms, bringing new hope to this disease that has long lacked effective treatments.
Trofinetide in Rett Syndrome
Rett syndrome is a rare genetic disorder that affects psychomotor development in children, mainly due to mutations in the methyl-binding protein 2 (MECP2) gene on the X chromosome. Rett syndrome is a neurodevelopmental disorder that affects female children and is characterized by loss of verbal communication, stereotyped hand movements, seizures, and behavioral problems. Trofinetide, through its synthetic glycine-proline-glutamate analogue properties, aims to improve the adverse pharmacokinetic profile of naturally occurring GPE in the brain, thereby positively affecting the symptoms of patients with Rett syndrome.
How does Trofinetide Work?
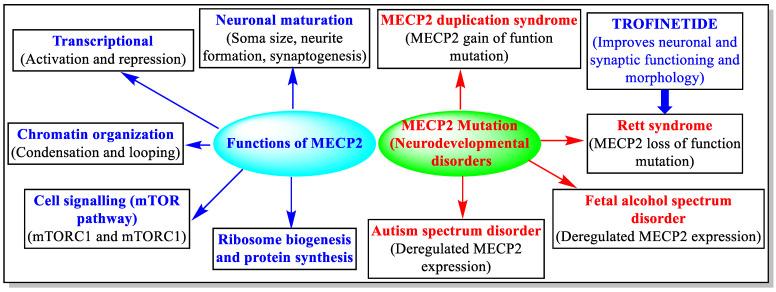
Rett syndrome is a rare inherited neurodevelopmental disorder whose core pathological mechanism is closely related to mutations in the MECP2 gene. The MECP2 gene plays an important role in brain development and is involved in regulating gene expression, synaptic function, and homeostasis of the nervous system. However, when the MECP2 gene is mutated, these functions are significantly weakened or lost, leading to synaptic abnormalities, increased neuroinflammation, and degeneration of nerve cells. These changes are the main cause of core symptoms such as speech impairment, motor coordination difficulties, and cognitive deterioration in patients with Rett syndrome.
As an innovative therapy for Rett syndrome, Trofinetide’s mechanism of action precisely targets the above-mentioned pathological processes, aiming to comprehensively improve the neurological function and symptomatic manifestations of patients. The following are the specific mechanisms by which Trofinetide works at multiple levels:
Improves Synaptic Function
Synapses are key sites for signal transmission between neurons, and their structural and functional integrity directly affect the normal functioning of brain function. In patients with Rett syndrome, synaptic plasticity is reduced and nerve signaling is inhibited due to defects in the MECP2 gene, ultimately leading to significant deterioration of cognitive and behavioral abilities. Trofinetide is able to promote normal signaling between neurons by enhancing synaptic plasticity. In addition, Trofinetide repairs damaged synaptic structures, radically improving the brain’s signaling network. This effect not only helps to restore the patient’s cognitive abilities, but also improves their language and social interaction skills.
Inhibits Inflammatory Responses
Neuroinflammation is one of the important pathological features of Rett syndrome, which is characterized by excessive release of inflammatory cytokines and abnormal activation of glial cells. The inflammatory response can cause further damage to neurons and exacerbate the neurological deterioration of the patient. Trofinetide is able to inhibit the inflammatory response through a variety of mechanisms. On the one hand, it can reduce the release of inflammatory cytokines such as IL-6 and TNF-α, thereby reducing the damage of inflammation to neurons. On the other hand, it is able to inhibit the overactivation of astrocytes and microglia and maintain the stability of the nervous system microenvironment. This effect helps to alleviate the neurodegenerative changes of the patient and creates favorable conditions for the restoration of neurological function.
Promotes Antioxidant Reactions
Oxidative stress is another significant pathological feature of Rett syndrome. Due to the excessive accumulation of free radicals, the patient’s nerve cells face severe oxidative damage, which can further weaken brain function. Trofinetide has a powerful antioxidant capacity, which can reduce the production of free radicals and promote the activity of antioxidant enzymes, thereby reducing the damage of oxidative stress to nerve cells. This antioxidant effect not only protects the structural integrity of nerve cells, but also slows the disease progression and provides patients with more stable support for nervous system function.
Cell Protection and Regeneration
The brains of patients with Rett syndrome are often accompanied by increased apoptosis and decreased regenerative capacity of nerve cells. Trofinetide is able to protect nerve cells from damage through a variety of mechanisms while promoting their regeneration. On the one hand, it can reduce the apoptosis of neurons and prolong the survival time of cells. On the other hand, it is able to increase levels of insulin-like growth factor 1. IGF-1 plays a key role in promoting neuronal growth and repair, providing regenerative support to damaged nerve cells, thereby enhancing the patient’s brain repair capacity. This cytoprotective and regenerative effect is the core foundation of Trofinetide’s improvement of Rett syndrome symptoms.
Comprehensive Multi-dimensional Improvement
Through the above mechanisms, Trofinetide can not only improve the core symptoms of patients with Rett syndrome, but also improve the functional stability of the nervous system as a whole. This multidimensional effect makes trofinetide a very promising treatment option, bringing hope to patients with Rett syndrome who have long lacked effective treatments.
Development of Trofinetide
The U.S. Food and Drug Administration (FDA) approved Trofinetide (brand name Daybue) in March 2023 for the treatment of Rett syndrome in adults and children 2 years of age and older. The approval is based on a Phase 3 clinical trial called Lavender, which evaluated the efficacy and safety of trofinetide in 187 women aged 5 to 20 years with Rett syndrome.
In a 12-week randomized, double-blind, placebo-controlled trial, the study evaluated the efficacy and safety of trofinetide in patients with Rett syndrome. The primary endpoints of the trial included change in the Rett Syndrome Behavior Questionnaire (RSBQ) total score, which assesses improvement in core behavioral symptoms, and the Clinical Global Impression of Improvement Scale (CGI-I) score, which measures change in the patient’s overall condition. The results showed that the Trofinetide group demonstrated a significant advantage over placebo in both primary endpoints, with significant improvements in communication, attention, and adaptive behavior, as well as a significantly higher proportion of physicians rating patients for overall symptom improvement, further confirming the clinical efficacy of Trofinetide.
Summary
Trofinetide marks an important breakthrough in this area as the first FDA-approved treatment specific to Rett syndrome. Its unique mechanism of action, multi-dimensional neuroprotective function and remarkable efficacy in clinical trials provide new hope for the treatment of patients with Rett syndrome.
While the approval of Trofinetide is a milestone, there are still areas to explore.
Long-term Efficacy and Safety: Larger, long-term studies are needed to validate the ongoing efficacy and potential side effects of trofinetide.
Individualized Treatment: To further study the differences in patients’ responses to Trofinetide to provide the best treatment options for patients with different genotypes or symptom characteristics.
Combination Therapy Possibilities: The effects of trofinetide in combination with other potential drugs or treatments deserve further discussion.
The advent of Trofinetide has ushered in a new era in the treatment of Rett syndrome and provided valuable experience for drug development for other rare neurodevelopmental disorders.
References
- Parent, Harrison, et al., Trofinetide: a pioneering treatment for Rett syndrome. Trends in Pharmacological Sciences (2023).
- Hudu, Shuaibu A., et al., Trofinetide for Rett syndrome: highlights on the development and related inventions of the first USFDA-approved treatment for rare pediatric unmet medical need. Journal of Clinical Medicine 12.15 (2023): 5114.