Intranasal (IN, intranasal) delivery of peptides has received considerable attention as a potential non-invasive delivery route through which peptide drugs can easily enter systemic circulation. Nasal delivery offers several advantages, including the relatively large absorbable surface area of the nasal cavity, the high permeability and vascular properties of nasal tissue, and the avoidance of gastric degradation and hepatic first-pass metabolism.
Currently, available peptide nasal spray products include the following products:
Calcitonin Salmon (trade name Miacalcin®)
The active ingredient in Miacalcin® is the peptide calcitonin salmon (salmon calcitonin), a calcitonin hormone found in salmon. Like human calcitonin, calcitonin salmon is a peptide hormone that is secreted by the parafollicular cells of the thyroid gland in response to hypercalcemia and reduces blood calcium and phosphorus by promoting renal excretion. Miacalcin® nasal spray is used to treat osteoporosis in women who have been menopausal for at least 5 years. It should work in conjunction with adequate calcium and vitamin D intake. Miacalcin® nasal spray is aimed at patients who are not candidates for alternative therapies, such as those for whom other therapies are unsuitable or those who are intolerant or unwilling to use other therapies.
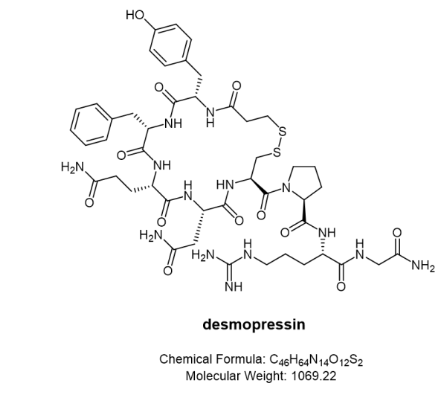
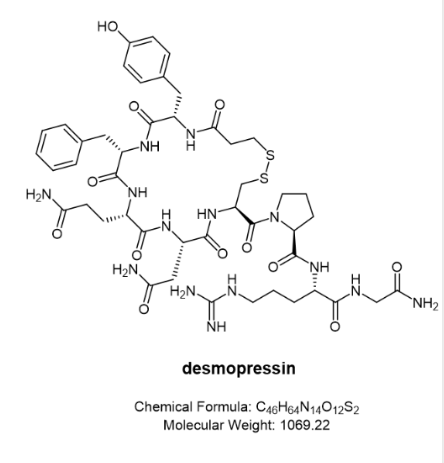
Desmopresin (trade name Stimate®)
Stimate® Nasal Spray is indicated for patients with haemophilia with coagulation factor VIII activity levels greater than 5% (mild). Stimate® Nasal Spray contains desmopressin acetate, a synthetic analog of the natural hormone Arginine vasopressin, as the active substance. A single (0.1 mL, 150 μg) spray of Stimate® nasal spray solution has an anti-diuretic activity of approximately 600 IU. The half-life of Stimate® Nasal Spray is between 3.3 and 3.5 hours and the intranasal dose ranges from 150 to 450 μg. The plasma concentration of Stimate® Nasal Spray reaches a maximum of approximately 40 to 45 minutes after administration. When administered by the intranasal route as a 1.5 mg/mL solution, the bioavailability of Stimate® nasal spray ranged from 3.3% to 4.1%.
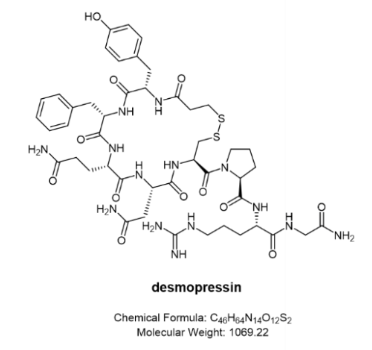
Desmopressin (trade name Octostim®)
Also desmopressin, its original developer Ferring Pharmaceuticals developed it as a different nasal spray. Octostim® is an anti-diuretic, it can be used to treat central insipidus, a condition in which the kidneys are unable to retain water due to a deficiency of antidiuretic hormone (ADH). This condition causes frequent urination and thirst. It is also used to treat temporary thirst and increased urination that may occur after a head injury or certain types of brain surgery. For these uses, Desmopressin Nasal Solution works by helping to reduce the amount of urine produced by the kidneys.
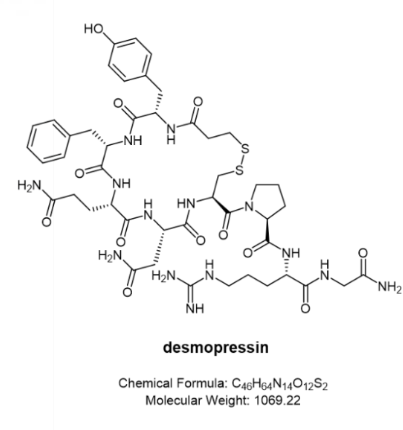
Desmopressin (trade name Minirin®)
Primary nocturnal enuresis: Minirin® nasal spray is indicated for the management of primary nocturnal enuresis. It can be used alone or as an adjunct to other non-pharmacological interventions for behavioral modification. It has been shown to be effective in certain cases that are difficult to treat with conventional therapy.
Central intracranial insipidus: Minirin® nasal spray is indicated for the treatment of central intracranial diabetes insipidus and for anti-diuretic replacement therapy for temporary polyuria following head trauma or surgery in the pituitary region. It is not effective in treating nephrogenic diabetes insipidus.
The Minirin® nasal spray compression pump delivers 0.1 mL (10 μg) of desmopressin acetate per spray. In addition to the nasal spray, Minirin® is also available in the form of an oral tablet.
Desmopressin (trade name DDAVP)
DDAVP contains desmopressin acetate as the active substance with an anti-diuretic effect and 1 mL (0.1 mg) of intranasal DDAVP is active at approximately 400 IU. The biphasic half-lives of intranasal DDAVP were 7.8 minutes and 75.5 minutes in the fast and slow phases respectively, compared to 2.5 and 14.5 minutes for the other form of lysine vasopressin. Thus, intranasal DDAVP produces a rapid antidiuretic effect and a long duration of action after each dose.
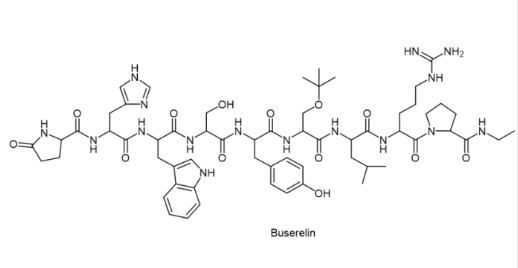
Buserelin (trade name Suprefact®)
Buserelin is used in men to treat advanced prostate cancer but is not a cure. Most types of prostate cancer require the male hormone testosterone to grow and spread. Buserelin works by reducing the amount of testosterone produced by the body. This action helps to slow or stop the growth of cancer cells and helps to relieve symptoms such as painful/difficult urination.
Buserelin is also used to treat endometriosis in women. It works by reducing the body’s production of the female hormone estrogen. This effect helps to shrink abnormal tissue and reduce the symptoms of endometriosis (e.g. pelvic pain, dysmenorrhoea).
Buserelin is an artificial hormone, similar to the natural hormone “gonadotropin-releasing hormone” (GnRH) produced by the human body. Although the use of buserelin promotes the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) (agonists), however, with prolonged administration of buserelin, the GnRH receptor becomes desensitized and completely stops responding to buserelin and endogenous GnRH. The profound desensitization of the GnRH receptor leads to a reduction in LH and FSH secretion from the anterior pituitary gland, followed by a cessation of gonadal sex hormone production, a significant reduction or loss of spermatogenesis in men, and an absence of ovulation in women, and the goal of “starving” cancer cells.
Suprefact contains 1.06 mg buserelin acetate (equivalent to 1 mg buserelin free base) per mL of the intranasal aqueous solution. For prostate cancer, the usual starting dose is 500 µg buserelin, by subcutaneous injection every 8 hours for 7 days. This is followed by maintenance therapy with a single daily injection of 200 µg buserelin or 400 µg of nasal spray delivered three times daily (2 sprays in each nostril). Women treated with buserelin for endometriosis are usually treated with buserelin nasal solution at a dose of 400 µg (2 sprays per nostril) 3 times a day. Treatment usually lasts for 6 months, but should not last longer than 9 months.
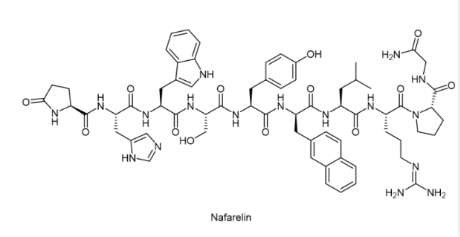
Nafarelin (trade name Synarel®)
Like buserelin, nafarelin is a GnRH (gonadotropin-releasing hormone) analog (agonist). It is used to treat endometriosis and precocious puberty. It is also used to treat uterine fibroids, to control ovarian stimulation in in vitro fertilization (IVF), and as part of cross-sex hormone therapy.
Synarel® is indicated for the treatment of central precocious puberty (CPP) (gonadotropin-dependent precocious puberty) in both male and female children. It is used as a nasal spray 2 to 3 times a day. Synarel® nasal solution contains nafarelin acetate (2 mg/mL). After filling the Synarel® pump, each start of the unit delivers a spray of approximately 100 μL containing approximately 200 μg nafarelin free base. The contents of a spray bottle are provided to deliver at least 60 sprays.
After intranasal administration, nafarelin acetate is rapidly absorbed into the systemic circulation. The maximum serum concentrations were reached between 10 and 40 minutes. The average peak concentration was 0.6 ng/mL (range 0.2 to 1.4 ng/mL), after a single dose of 200 μg free base nafarelin and after a single dose of 400 μg free base nafarelin, the mean peak concentration observed was 1.8 ng/mL (range 0.5 to 5.3 ng/mL). The bioavailability of the 400 μg dose averaged 2.8% (range 1.2 to 5.6%). The mean serum half-life of nafarelin after intranasal administration is about 3 hours. Approximately 80% of nafarelin acetate is bound to plasma proteins at 4°C.[3]
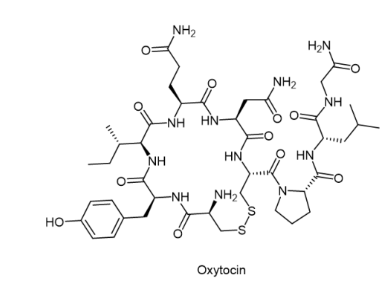
Oxytocin (trade name Syntocinon)
The uses of oxytocin nasal spray range from treating anxiety disorders to autism. In clinical trials, oxytocin has shown promise in the treatment of obesity and may have other super-indicated uses. It is a naturally occurring hormone that can also be used as a supplement.
Semax
Semax peptides are known for their neuroprotective, educational, and neurorestorative properties. Traditionally, Semax has been used to treat depression and anxiety. Semax was originally developed based on the molecular structure of ACTH (Adrenocorticotropic Hormone). Ongoing research suggests that Semax may be useful for individuals with cognitive disorders (including dementia, stroke, ADHD, etc.) and may better improve mental clarity and concentration, reduce anxiety and depressive symptoms, improve the clarity of thought and focus, and reduce the effects of stress, hence Semax is given the free translation of “brain peptide” in Chinese. “Semax is used for a wide range of indications, and in the medical setting, Semax has been prescribed for the treatment of anxiety disorders, ischaemic events, stroke, neuroregeneration, ADHD, opioid withdrawal, Parkinson’s disease, Alzheimer’s disease, thrombosis, and gastric disorders. It is worth noting that Cerebrolysin is mainly used in countries such as Russia and Ukraine and is not approved in most countries around the world.
Advantages of intranasal delivery of peptide drugs
Potential nose-to-brain route: The blood-brain barrier (BBB) is a major barrier to the delivery of therapeutic agents to the central nervous system (CNS), as most large molecular weight substances are severely restricted from crossing the BBB under normal circumstances. For biological agents, such as peptides, proteins, monoclonal antibodies, oligonucleotides, and gene and cell therapies via the nasal-brain pathway, nasal delivery offers a potential strategy to bypass the BBB and reach the brain, thereby providing new therapeutic approaches for indications such as Alzheimer’s disease, Parkinson’s disease, and antipsychotic drug-induced symptoms.
High efficacy and fast onset: Most biologicals are readily degraded enzymatically in the gastrointestinal tract, therefore the most common route of administration is by injection. However, when drugs are administered by the intranasal route, they enter through the respiratory zone around the inferior turbinate, where the nasal mucosa in the respiratory zone is richly vascularised and lined with columnar epithelial cells with a large surface area (> 150 cm2 ), which is conducive to drug absorption and has strong permeability. [5] As the intranasal route avoids enzymatic degradation in the gastrointestinal tract and first-pass metabolism in the liver, as a result, it reduces the common barriers that limit drug absorption and facilitates the rapid onset of action.
High patient dependence: Intranasal delivery will undoubtedly result in higher patient compliance compared to injection. Good patient compliance is important for therapeutic outcomes, especially for intranasal drug products, which must be administered regularly and consistently to ensure sustained therapeutic outcomes. Patients’ satisfaction and comfort with the administration of medication is highly dependent on their compliance. The intranasal route is both non-invasive and highly tolerable, expanding the possibilities and compliance of patient self-administration
References
- Fosgerau, K. et al. Peptide therapeutics: current status and future directions. Drug Discov. Today. 2015, 20, 122-128.
- Arora, P. et al. Permeability issues in nasal drug delivery. Drug Discov. Today. 2002, 7, 967-975.
- Darshana, S. et al. Nasal Delivery of Proteins and Peptides. Glob. J. Pharm. Sci. 2017, 1, 555569.
- Grassin-Delyle, S. et al. Intranasal drug delivery: an efficient and non-invasive route for systemic administration: focus on opioids. Pharmacol. Ther. 2012, 134, 366-379.
- Ward, D. Optimising Nasal Drug Products for Systemic Delivery.
- Bakri, W. A., et al. Overview of intranasally delivered peptides: key considerations for pharmaceutical development. Expert Opinion on Drug Delivery. 2018, 15, 991-1005.
- Basu, S. et al. Preparation and characterization of mucoadhesive nasal gel of venlafaxine hydrochloride for treatment of anxiety disorders. Indian J Pharm Sci. 2012, 74, 428-433.