The effect of drugs is achieved by interfering with the pathogenic process by binding between drug molecules and receptors. The binding between the drug and the receptor protein, in addition to the weak and unstable non-covalent binding, can also be achieved by a more solid covalent bond. The latter strategy has spawned a new trend of drug molecular design in the 21st century-covalently bonded drugs. Covalent peptide drugs approved by the FDA since 2011 (Tab 1.) have focused on the treatment of hepatitis C.
Tab 1. FDA-approved covalent peptide drugs from 2011 to 2019
| Name | Therapeutic Field | Active Gene | Chemical Structure |
| Telaprevir
|
Anti-hepatitis C virus | α-ketoamide | 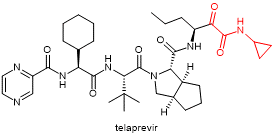 |
| Boceprevir
|
Anti-hepatitis C virus | α-ketoamide | 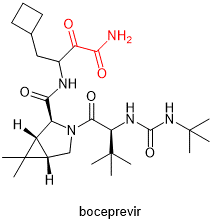 |
| Narlaprevir
|
Anti-hepatitis C virus | α-ketoamide | 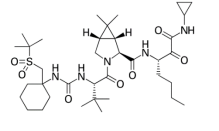 |
Covalent peptide drugs usually have electrophilic groups (because the reactive groups on the corresponding protein receptor are generally nucleophilic groups), and the reaction between the electrophilic group and the nucleophilic group of the receptor leads to covalent binding between the drug molecule and the receptor, thus achieving the therapeutic effect. The invention of covalent peptide drugs has led to the re-emergence of many protein receptors previously considered “undruggable”.
One of the advantages of covalent peptide drug development is that it can combine molecules or groups based on the previous results of non-covalent drugs and introduce active groups into the lead of non-covalently bonded peptides that bind closely to the target protein. When non-covalent binding occurs, the active groups are introduced into the vicinity of the target amino acid residue of the target protein in the three-dimensional space. In this case, the amino acid residue reacts irreversibly with the active groups on the drug molecule to achieve the ultimate goal of inhibiting the target protein (Fig 1).
Fig. 1. The covalent binding of a peptide drug to a target protein based on non-covalent interaction
From a protein receptor point of view, cysteine is the most studied target for drug designers because its sulfhydryl group acts as an excellent nucleophile. In the process of drug design, acrylamide groups are often attached to the lysine residues in the original non-covalent peptide drugs, and covalent bonds are formed by the reaction between the sulfhydryl group of the receptor cysteine and the acrylamide of the drug molecules. A study showed that a modified peptide drug (a 13-peptide containing acrylamide lysine) was more effective at inhibiting Siah (E3 ubiquitin-protein ligase) and that the peptide drug was specifically bound to cysteines at key sites. However, when the acrylamide group is replaced by the more active chloroacetamide group, the resulting peptide drug loses its cysteine selectivity and can instead react with other cysteines on the Siah protein receptor. This result confirmed that the reactivity of the electrophilic groups of covalent drugs is very important for drug selectivity.
Since cysteine is scarce in native protein receptors, covalent peptide drugs targeting lysine and histidine have gained attention. In one study, a peptide drug with a benzoyl fluoride group formed a strong covalent binding to its receptor p53-MDM2/4 protein, effectively inhibiting the activity of this protein. The activity of the modified peptide was 5-fold enhanced compared with that of the peptide precursor without benzenesulfonyl fluoride. Notably, the SuFEx reaction between benzenesulfonyl fluoride and the amino group of the lysine side chain is considered a new type of click reaction.
Encouraged by the sulfur-fluorine exchange reactions, different active groups of benzenesulfonyl fluoride and fluorosulfonic acid aromatic esters were of interest. They can covalently bind to lysine, tyrosine, and histidine.
In the search for drugs to treat COVID-19, covalent peptide drugs have also emerged. A peptide molecule named VIR251, containing methyl acrylate structure, can covalently interact with papain-like protease (PLpro) of SARS-CoV-2 protein mediating COVID-19 through Michael’s addition. Thus, the physiological function of the protein was inhibited.
Conclusion
With the continuous development of small molecule covalent drugs and the R&D of non-covalent peptide drugs, the resources available for the combination of the two products, covalent peptide drugs, will continue to expand, and promote the R&D and marketing of new drugs in this field. However, while covalently bonded drugs have the advantage of long duration and low dose, practitioners are still concerned about the unanticipated side effects of these active substances indiscriminately binding to proteins in the body, as well as the possible immune response to these drugs. Therefore, safety has become a top priority in the design and development of covalently bonded drugs.
Related Products:
| Name | Description |
| Therapeutic Peptides | Creative Peptides is the world’s first-choice supplier of peptides used as pharmaceutical agents with a range of more than 100 peptides and related products. |
| Cysteine | Cysteine is a semiessential proteinogenic amino acid with the formula HOOC-CH-(NH2)-CH2-SH. |
| Lysine | Lysine is an α-amino acid that is a precursor to many proteins. |
| Histidine | Histidine is an essential α-amino acid that is used in the biosynthesis of proteins. |
| Tyrosine | Tyrosine is a type of amino acid, which are the building blocks of protein. |
References
[1] Dong J, Krasnova L, Finn MG, et al. Sulfur (VI) Fluoride Exchange (SuFEx): Another Good Reaction for Click Chemistry. Angewandte Chemie Int Ed. 2014, 53,9430-9448.
[2] Hoppmann C, Wang L. Proximity-enabled bioreactivity to generate covalent peptide inhibitors of p53-Mdm4. Chem. Commun. 2016, 52, 5140-5143.
[3] Pietro Delre, Fabiana Caporuscio, Michele Saviano, Giuseppe Felice Mangiatordi, Repurposing Known Drugs as Covalent and Non-covalent Inhibitors of the SARS-CoV-2 Papain-Like Protease. J. Med. Chem. 2019, 62, 9188-9200.
[4] Stebbins JL, Santelli E, Feng Y, et al. Structure-Based Design of Covalent Siah Inhibitors. Chem Biol. 2013, 20, 973-982.
[5] Wang N, Yang B, Fu C, et al. Genetically Encoding Fluorosulfate-l-tyrosine To React with Lysine, Histidine, and Tyrosine via SuFEx in Proteins in Vivo. J. Am. Chem. Soc. 2018, 140, 4995-4999.